|
Dynamic Phosphorylation of CENP-A at Ser68 Orchestrates Its Cell-Cycle-Dependent Deposition at Centromeres
Zhouliang Yu1, 2, 9, Xiang Zhou1, 9, Wenjing Wang1, Wenqiang Deng1, 2, Junnan Fang1, 2, Hao Hu1, 2, Zichen Wang1, Shangze Li3, Lei Cui1, 4, Jing Shen1, Linhui Zhai5, Shengyi Peng2, 6, Jiemin Wong7, Shuo Dong8, Zengqiang Yuan6, Guangshuo Ou1, Xiaodong Zhang3, Ping Xu5, Jizhong Lou4, Na Yang1, Ping Chen1, Rui-Ming Xu1, Guohong Li1
1 National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 College of Life Sciences, Wuhan University, Wuhan, Hubei 430072, China
4 Laboratory of Noncoding RNAs, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China
5 State Key Laboratory of Proteomics, Beijing Proteome Research Center, Beijing Institute of Radiation Medicine, Beijing 102206, China
6 State Key Laboratory of Brain and Cognitive Sciences, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China
7 Institute of Biomedical Sciences and School of Life Sciences, East China Normal University, Shanghai 200241, China
8 Department of Medicine, Baylor College of Medicine, Houston, TX 77030, USA
Abstract
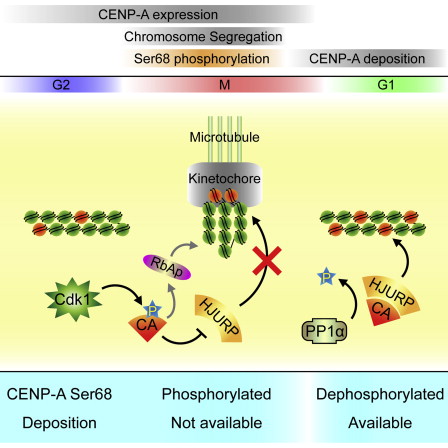
The H3 histone variant CENP-A is an epigenetic marker critical for the centromere identity and function. However, the precise regulation of the spatiotemporal deposition and propagation of CENP-A at centromeres during the cell cycle is still poorly understood. Here, we show that CENP-A is phosphorylated at Ser68 during early mitosis by Cdk1. Our results demonstrate that phosphorylation of Ser68 eliminates the binding of CENP-A to the assembly factor HJURP, thus preventing the premature loading of CENP-A to the centromere prior to mitotic exit. Because Cdk1 activity is at its minimum at the mitotic exit, the ratio of Cdk1/PP1α activity changes in favor of Ser68 dephosphorylation, thus making CENP-A available for centromeric deposition by HJURP. Thus, we reveal that dynamic phosphorylation of CENP-A Ser68 orchestrates the spatiotemporal assembly of newly synthesized CENP-A at active centromeres during the cell cycle.
Graphical Abstract
See more about this article: http://www.sciencedirect.com/science/article/pii/S1534580714007692# |